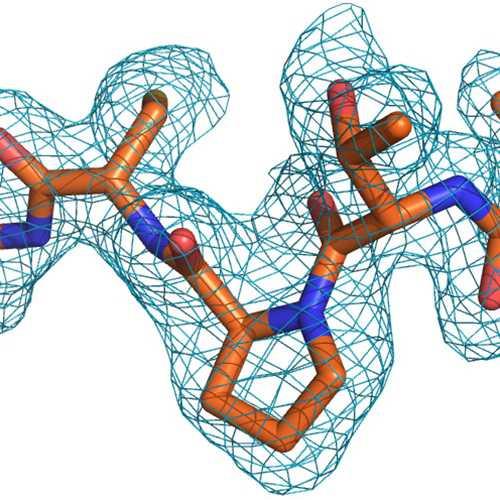
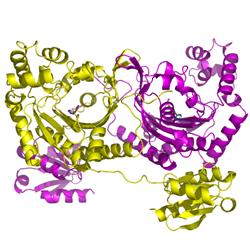
产品安全信息
Francklyn, C. and Schimmel, P., Enzymatic aminoacylation of an eight-base-pair microhelix with histidine. PNAS, 87, 8655-8659, (1990). Franckyln, C., Harris, D., and Moras, D., Crystallization of Histidyl-tRNA Synthase from Escherichia coli, Journal of Molecular Biology, 241, 275-277, (1994). Yan, W. and Francklyn, C. Cytosine 73 is a discriminator nucleotide in vivo for histidyl-tRNA in Escherchia coli. Journal of Biological Chemistry, 269:13, 10022-10027 (1994). Bovee, M.L., Yan, W., Sproat, B.S., and Francklyn, C.S. tRNA discrimination at the binding step by a class II aminoacyl-tRNA synthetase. Biochemistry, 38: 41, 13725-13735 (1999). Arnez, J.G., Harris, D.C., Mitschler, A., Reese, B., Francklyn, C.S., and Moras, D. Crystal structure of histidyl-tRNA synthetase from Escherichia coli complexed with histidyl-adenylate. EMBO Journal, 14:17, 4143-4155 (1995). Yan, W., Augustine, J., and Francklyn, C. A tRNA identity switch mediated by the binding interaction between a tRNA anticodon and the accessory domain of a class II aminoacyl-tRNA synthetase. Biochemistry, 35:21, 6559-6568 (1996). Arnez, J.G., Augustine, J.G., Moras, D., and Francklyn, C. The first step of aminoacylation at the atomic level in histidyl-tRNA synthase. PNAS, 94, 7144-7149 (1997). Francklyn, C., Adams, J., and Augustine, J. Catalytic defects in mutants of class II histidyl-tRNA synthetase from Salmonella typhimurium previously linked to decreased control of histidine biosynthesis regulation. Journal of Molecular Biology, 280, 847-858 (1998). Francklyn, C., Musier-Forsyth, K., and Martinis, S.A. Aminoacyl-tRNA synthetases in biology and disease: new evidence for structural and functional diversity in an ancient family of enzymes RNA, 3, 945-960 (1997). Hawko, S.A., and Francklyn, C.S. Covariation of a specificity-determining structural motif in an aminoacyl-tRNA synthetase and a tRNA identity element. Biochemistry, 40:7, 1930-1936 (2001). Connoly, S.A., Rosen, A.E., Musier-Forsyth, K., and Francklyn, C.S. G-1:C73 recognition by an arginine cluster in the active site of Escherichia coli histidyl-tRNA synthetase. Biochemistry, 43: 4, 962-969 (2004). Guth, E., Connoly, S.H., Bovee, M., and Francklyn, C.S. A substrate-assisted concerted mechanism for aminoacylation by a class II aminoacyl-tRNA synthetase. Biochemistry, 44:10, 3785-3794 (2005) Guth, E. and Francklyn, C.S. Kinetic discrimination of tRNA identity by the conserved motif 2 loop of a class II aminoacyl-tRNA synthetase. Molecular Cell, 25, 531-542 (2007). Francklyn, C.S., First, E.A., Perona, J.J., and Hou, Y. Methods for kinetic and thermodynamic analysis of aminoacyl-tRNA synthetases. Methods, 44:2, 100-118 (2008). If you publish research with this product, please let us know so we can cite your paper.